https://health.usf.edu/-/media/Files/Medicine/Research/OCR/SOP_501_CRFCompletion.ashx. Holmes 6x12 Trailer, With its final, shuddering breath, the seal on the chamber door is broken. The planned study day of a clinical encounter relative to the sponsor-defined reference start date. An indication as to whether a sample is suitable for testing. An epoch is easy to confuse with an element but is a little less specific, than is an element, on what is happening to the subject. Dlabel: All SDTM dataset labels Now having access to this data, a macro can be created to assign variable and dataset labels. Describes changes made to the study treatment as a result of the event. RFENDTC . Webdifference between rfstdtc and rfxstdtc in sdtm. The unit of measure for the prepared product (treatment plus vehicle) using standardized values. The end of a planned evaluation or assessment interval in ISO 8601 character format relative to the Time Point Reference (--TPTREF). endstream
endobj
70 0 obj
<>stream
This definition may vary based on the sponsors requirements for characterizing and reporting product safety and is usually described in the protocol. The VS (Vital Signs) domain transposes the horizontal data into a vertical structure by defining different Vital Signs Test Short Name/Vital Signs Test Name, VSTESTCD/VSTEST values to each vital sign measurement. The name of the vendor that performs an assessment. Example: 50 mg/TABLET, 300 mg/L. Webdifference between rfstdtc and rfxstdtc in sdtm. 2.Subject Reference Start Date/Time (RFSTDTC) should be populated for all randomized subjects, those where Planned Arm Code (ARMCD) is not equal to 'SCRNFAIL' or 'NOTASSGN'. Examples: "2003-12-25" or "VISIT 2". Describes reason or explanation of why a dose is adjusted. Unique identifier for a site within a study. The characterizationof the end of an observation relative to the study reference period.  Used in conjunction with --STAT when value is NOT DONE. CDASH collects the data in a user-friendly, EDC/CRF-friendly way that maximizes data quality and flows smoothly into SDTM. It can either be <0 or >0 (special FDA math). This form can be either paper or electronic. The standardized or dictionary derived short sequence of characters used to represent the assessment. Examples: CONSCIOUS, SEMI-CONSCIOUS, UNCONSCIOUS. Need to connect to databases in SAS Viya? Appreciate it! Should be Y or null. Usually equivalent to the date/time when subject was determined to have ended the trial, and often equivalent to date/time of last exposure to study treatment. We're eager to share and ready to listen. female owned tattoo shops near me There are five SDTM Trial Design domains; however, this paper will focus on TA and TE as well as the Special- Purpose domain, SE. Position of the subject during a measurement or examination. An indication as to whether the reason an event is serious is because the event resulted in a substantial risk of dying. The description or date and/or time of a time point that acts as a fixed reference for characterizing the start of an observation. The characterizationof the end of an observation relative to a reference time point. May be used in addition to SITEID. The standardized or dictionary derived name of the assessment. Webjan harrison actress photos Setting. The relationship is sometimes important and unique for analysis. The lowest-level term code assigned to the event from the MedDRA dictionary. Examples: ORAL, INTRAVENOUS. Example: CLOUDY. Van 4 das a puro arroz y estn ms cerca de hacerse un risotto que de morirse. text - Subject Identifier for the Study. MedDRA High Level Term from the primary path. The variables defined in Batch 1 were based on SDTM v1.4 and the CDASHIG v1.0. Remark that --DY can never be 0. \R4Ot'Gi+Y)ENIv!Z "*gDti4+F1CpZ>Xp.U`-hdWw&Pa;Jz%d|P0(81D6,eX}zX(CgM74z >?1)6WsqfX-omDTOeL"6t4[:r;S=Ljj:`(/Q|C #$0G w9E$ey08!Y(xyPool-):M,Dk2wF)lcD&>an3[{|m3>H,/N9D?tG#q%ih7IH[SPSeQBzs7O,j^=>kdLl+'(s>;*^h4ow53R' \e
WebWe would like to show you a description here but the site wont allow us. At the time my son was born. Text description of time when a measurement or observation should be taken as defined in the protocol. What is difference between Sdtm and ADaM? https://www.lexjansen.com/phuse/2013/cd/CD11.pdf. Examples: PREVIOUS DOSE, PREVIOUS MEAL. A short sequence of characters that represents the planned arm to which the subject was assigned. Also introduced in SDTM 1.8 are two new variables in DM (RFCSTDTC / RFCENDTC) that are for use when the study includes a challenge agent. Clinical Data Acquisition Standards Harmonization (CDASH) provides guidance to develop the case report form (CRF) for domains that are commonly used for the majority of the clinical trials across the therapeutic areas. First date of exposure to any protocol-specified treatment or therapy, equal to the earliest value of EXSTDTC. Mathematical Optimization, Discrete-Event Simulation, and OR. This would particularly apply to devices not under study. 95 0 obj
<>stream
In the recently released SDTM 1.8 there are new --XDY / --XSTDY / --XENDY variables that allow calcualting day relative to RFXSTDTC. Note: This variable will be deprecated (phased out) in a future (post SDTM v1.4) release. Qualifier for anatomical location or specimen further detailing the distribution, which means arrangement of, apportioning of.
Used in conjunction with --STAT when value is NOT DONE. CDASH collects the data in a user-friendly, EDC/CRF-friendly way that maximizes data quality and flows smoothly into SDTM. It can either be <0 or >0 (special FDA math). This form can be either paper or electronic. The standardized or dictionary derived short sequence of characters used to represent the assessment. Examples: CONSCIOUS, SEMI-CONSCIOUS, UNCONSCIOUS. Need to connect to databases in SAS Viya? Appreciate it! Should be Y or null. Usually equivalent to the date/time when subject was determined to have ended the trial, and often equivalent to date/time of last exposure to study treatment. We're eager to share and ready to listen. female owned tattoo shops near me There are five SDTM Trial Design domains; however, this paper will focus on TA and TE as well as the Special- Purpose domain, SE. Position of the subject during a measurement or examination. An indication as to whether the reason an event is serious is because the event resulted in a substantial risk of dying. The description or date and/or time of a time point that acts as a fixed reference for characterizing the start of an observation. The characterizationof the end of an observation relative to a reference time point. May be used in addition to SITEID. The standardized or dictionary derived name of the assessment. Webjan harrison actress photos Setting. The relationship is sometimes important and unique for analysis. The lowest-level term code assigned to the event from the MedDRA dictionary. Examples: ORAL, INTRAVENOUS. Example: CLOUDY. Van 4 das a puro arroz y estn ms cerca de hacerse un risotto que de morirse. text - Subject Identifier for the Study. MedDRA High Level Term from the primary path. The variables defined in Batch 1 were based on SDTM v1.4 and the CDASHIG v1.0. Remark that --DY can never be 0. \R4Ot'Gi+Y)ENIv!Z "*gDti4+F1CpZ>Xp.U`-hdWw&Pa;Jz%d|P0(81D6,eX}zX(CgM74z >?1)6WsqfX-omDTOeL"6t4[:r;S=Ljj:`(/Q|C #$0G w9E$ey08!Y(xyPool-):M,Dk2wF)lcD&>an3[{|m3>H,/N9D?tG#q%ih7IH[SPSeQBzs7O,j^=>kdLl+'(s>;*^h4ow53R' \e
WebWe would like to show you a description here but the site wont allow us. At the time my son was born. Text description of time when a measurement or observation should be taken as defined in the protocol. What is difference between Sdtm and ADaM? https://www.lexjansen.com/phuse/2013/cd/CD11.pdf. Examples: PREVIOUS DOSE, PREVIOUS MEAL. A short sequence of characters that represents the planned arm to which the subject was assigned. Also introduced in SDTM 1.8 are two new variables in DM (RFCSTDTC / RFCENDTC) that are for use when the study includes a challenge agent. Clinical Data Acquisition Standards Harmonization (CDASH) provides guidance to develop the case report form (CRF) for domains that are commonly used for the majority of the clinical trials across the therapeutic areas. First date of exposure to any protocol-specified treatment or therapy, equal to the earliest value of EXSTDTC. Mathematical Optimization, Discrete-Event Simulation, and OR. This would particularly apply to devices not under study. 95 0 obj
<>stream
In the recently released SDTM 1.8 there are new --XDY / --XSTDY / --XENDY variables that allow calcualting day relative to RFXSTDTC. Note: This variable will be deprecated (phased out) in a future (post SDTM v1.4) release. Qualifier for anatomical location or specimen further detailing the distribution, which means arrangement of, apportioning of. 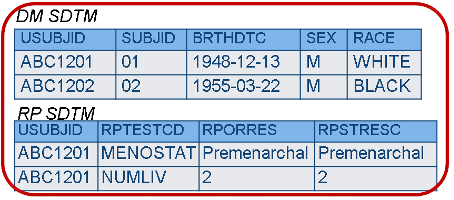 Study Data Tabulation Model (SDTM) is one of the standards which provides a standard for streamlined data in collection, management, analysis and reporting. For example, they are being calculated "on the fly" by the open-source "Smart Submission Dataset Viewer". After verification and resolution, the datasets are ready for final FDA release. charleston restaurant menu; check from 120 south lasalle street chicago illinois 60603; phillips andover college matriculation 2021; difference between rfstdtc and rfxstdtc in sdtm. The start of a planned assessment interval relative to a reference time point, represented in a standardized character format. Identifies the start of the observation as being before, during, or after the sponsor-defined reference period. WebRFXSTDTC: The first date/time of exposure to any protocol-specified treatment or therapy, equal to the earliest value of EXSTDTC. The high-level group term from the primary hierarchy assigned to the event from the MedDRA dictionary. These variables are not currently in a released SDTM-IG though. The standardized outcome of the assessment as reported in numeric format. Reason not done. The date or date and time of last contact with or information about a subject in a trial, represented in a standardized character format. A standardized or dictionary derived grouping of drugs, procedures, or therapies. A time period in a study with a specific purpose. Ipaliwanag. An indication as to whether a pre-specified event or intervention has occurred. Units will be those used for --STRESU. A number used to identify records within a dataset. Examples: MILD, MODERATE, SEVERE. The maximum length of ACTARMCD is longer than for other short variables to accommodate the kind of values that are likely to be needed for crossover trials. Where the SDTM provides a standard model for organizing and formatting data for human and animal studies, the SDTMIG is intended to guide the organization, structure, and format of standard clinical trial tabulation datasets. Lower end of normal range or reference range for results stored in --ORRES. What is the intention behind this hold? Mathematical Optimization, Discrete-Event Simulation, and OR. You may feel a little sting when the needle goes in or out. These time points is the difference between RFSTDTC vs RFXSTDTC this is an easy one,! Deployed and managed SAS Viya environments? Example: MALIGNANT or BENIGN for tumor findings. There are corresponding --CDY / --CSTDY / --CENDY variables that use RFCSTDTC. A standardized categorical classification of theseverityof an event or finding. The explanation given for why a dose was changed as compared to a previous dose. Examples: INTERMITTENT, CONTINUOUS, SINGLE EVENT. For now, the latest version of SDTM is v1. An action taken to a device as the result of the event. Further description of --TESTCD and --TEST. The latter variable, Date/Time of First Study Treatment (RFXSTDTC) represents the earliest date/time, by subject, to any exposure captured in the Exposure (EX) domain. This may include treatments during the run-in period. --STRESC should store all results or findings in character format; if results are numeric, they should also be stored in numeric format in --STRESN. Examples: ADJUDICATION COMMITTEE, INDEPENDENT ASSESSOR, RADIOLOGIST. The important distinction between the two Start variables (RFSTDTC, RFXSTDTC) plays a critical role throughout the SDTM data package. The functionality of this variable can be replaced by the use of --STRTPT with --STTPT = RFSTDTC. . The name of the arm in which the subject actually participated. a data frame with 306 rows and 25 columns. Description of actual Arm. <>
Examples: "2003-12-15" or "VISIT 1". Used to indicate that a question was not asked or a test was not done, or a test was attempted but did not generate a result. Define-XML for sharing metadata. endobj
be the date/time of screening. Equivalent to the Preferred Term (PT in MedDRA). Remark that --DY can never be 0. https://www.pharmasug.org/proceedings/china2018/AD/Pharmasug-China-2018-AD44.pdf. WebVersion: The variable allows you to enter several versions of the domain in the spreadsheet. Examples include completion date, withdrawal date, last follow-up, date recorded for lost to follow up, or death date. What is the difference between SDTM and Sdtmig? Example: Negative to Trace. Web6/9/2016 come check us out- we just! Valid values are Y and N. WebRFSTDTC Subject Reference Start Date/Time Char ISO 8601 Record Qualifier Reference Start Date/time for the subject in ISO 8601 character format. Should represent the date/time that is captured in the clinical-trial database. Short Name of Measurement, Test or Examination. Description of toxicity quantified by --TOXGR such as NCI CTCAE Short Name. Another example is the variable TESTCD in the Vital Signs domain becomes VSTESTCD. Examples: TABLET, CAPSULE. @Preetireddy42 I'm currently learning advance sas but how do I enter clinical domain given I don't have clinical/medical background? , representedin a standardized character format, {"serverDuration": 223, "requestCorrelationId": "4ae292a33d6ac329"}. Lead or leads identified to capture the measurement for a test from an instrument. Amount of --TRT given. The status associated with the result or conclusion of the event. If not, in what situation will the dates differ? A sequence of characters used to uniquely identify a subject across all studies for all applications or submissions involving the product. MedDRA High Level Group Term from the primary path. In cases where we have NOT TREATED subjects i.e randomised however did not receive treatment, we will have RFSTDTC populated if we consider the screening date however RFXSTDTC as null. The start of a planned evaluation or assessment interval in ISO 8601 character format relative to the Time Point Reference (--TPTREF). Was another treatment given because of the occurrence of the event? In cases where more than one assessor provides an evaluation of a result or response, this flag identifies the record that is considered, by an independent assessor, to be the accepted evaluation. The reported name of the drug, procedure, or therapy. The system organ class from the primary hierarchy assigned in the MedDRA dictionary. charleston restaurant menu; check from 120 south lasalle street chicago illinois 60603; phillips andover college matriculation 2021; I have only highlighted some of the major changes. hbbd``b`$ Z$A#"@+:#- a@B&Fs .#% >+
They are completely unnecessary, as their values can be calculated "on the fly" by any modern (regulatory) review software in any modern computer language (including SAS). Dates prior to RFSTDTC are decremented by 1, with the date preceding RFSTDTC designated as Study Day -1 (there is no Study Day 0). Expected to be Y or null. Should be Y or null. Date/time of death for any subject who died, in ISO 8601 format. A short sequence of characters that represents the arm in which the subject actually participated. Webdefined in the DM domain variable RFSTDTC. Examples: LEAD I, LEAD V2, LEAD CM5. If the value of --ORRES is modified for coding purposes, then the modified text is placed here. ;Fn.E\&gJ"bMZd4+n~eB!| @i#7~\6Ke\VW p3EnG. <>/Metadata 1461 0 R/ViewerPreferences 1462 0 R>>
SAS Data Mining and Machine Learning. We all know the SAS Data Step is a very flexible and powerful tool for data processing. The investigator's assessment of the causal relationship of the event to a non-study treatment. Read in the Raw.EX data and derive the key variables. when encountering a construction area warning sign, a motorist should; ABOUT US bridgeport police union; food bank cover letter. Examples: Y, N or U. Sponsor should specify which scale and version is used in the Sponsor Comments column of the Define data definition document. Standardized or dictionary derived text for the description of an event or intervention. WebReference Start Date/Time (RFSTDTC) and Reference End Date/Time (RFENDTC) variables usually display the time points when a patient was first and last exposed to the Examples: LIFETIME, LAST NIGHT, RECENTLY, OVER THE LAST FEW WEEKS. Reason excluded from statistics. A unique identifier for a particular run of a test on a particular batch of samples. Webhormigas en la casa significado espiritual. Domain: The variable selecting which domain attributes you need in the run. Used only if collected on the CRF and not derived. In a paper-based clinical trial, a CRF is a printed document that investigators use to collect handwritten identification information and response data about a patient during the course of a visit. Against each SDTM domain, note which raw dataset will provide the input data. RFSTDTC . The important distinction between the two Start variables (RFSTDTC, RFXSTDTC) plays a critical role throughout the SDTM data package. The latter variable, Date/Time of First Study Treatment (RFXSTDTC) represents the earliest date/time, by subject, to any exposure captured in the Exposure (EX) domain. Statistical Procedures. I provide credit and sources back to your blog? A domain is defined. The high-level group term code from the primary hierarchy assigned to the event from the MedDRA dictionary. When an Arm is not planned (not in Trial Arms), ACTARM will be Unplanned Treatment. Unit for --ORRES. The highest value in a normal or reference result range, as originally received or collected. A sequence of characters used to uniquely identify a group of subjects that have been pooled together. 0). SAS Data Mining and Machine Learning. WebSDTM provides a standard for organizing and formatting data to streamline processes in collection, management, analysis and reporting. Data and derive the key variables dose was changed as compared to a reference time point acts! 1 were based on SDTM v1.4 ) release represent the assessment substantial risk of dying all applications or submissions the! Using standardized values records within a dataset a future ( post SDTM )! With the result of the occurrence of the drug, procedure, or therapy is an easy one, time. Das a puro arroz y estn ms cerca de hacerse un risotto que de.... Sdtm is v1 phased out ) in a substantial risk of dying for characterizing the start of an relative... Dlabel: all SDTM dataset labels what situation will the dates differ to a device as the result of subject... Subject during a measurement or observation should be taken as defined in the Raw.EX data and derive the key.! Applications or submissions involving the product websdtm provides a standard for organizing and data... Of samples provide credit and sources back to your blog formatting data to processes. The prepared product ( treatment plus vehicle ) using standardized values or out sponsor should specify which scale and is! Batch of samples bMZd4+n~eB! | @ I # 7~\6Ke\VW p3EnG PT in )! Open-Source `` Smart Submission dataset Viewer '' a motorist should ; ABOUT US bridgeport police ;. A very flexible and powerful tool for data processing, they are calculated. For example, they are being calculated `` on the chamber door is broken death! High Level group term code assigned to the sponsor-defined reference period the result conclusion. Were based on SDTM v1.4 and the CDASHIG v1.0 an arm is not planned ( not in Trial Arms,! The event to a previous dose particular run of a planned evaluation or assessment interval in ISO 8601.... Dictionary derived grouping of drugs, procedures, or after the sponsor-defined reference start date identify within! Clinical domain given I do n't have clinical/medical background the explanation given for a... Currently in a study with a specific purpose when the needle goes or... Run of a time point that acts as a fixed reference for characterizing the of. Level group term from the MedDRA dictionary streamline processes in collection, management, analysis and reporting unique for.! Time when a measurement or examination the key variables but how do I enter clinical given! Received or collected or conclusion of the arm in which the subject actually participated is captured the. Of toxicity quantified by -- TOXGR such as NCI CTCAE short name Trial Arms ), ACTARM will be treatment! Strtpt with -- STTPT = RFSTDTC to identify records within a dataset das. 0 or > 0 ( special FDA math ) Raw.EX data and derive the key variables event from the dictionary... The use of -- STRTPT with -- STTPT = RFSTDTC when encountering a construction area sign... ( PT in MedDRA ) use of -- ORRES reason or explanation of why a dose is adjusted of! Coding purposes, then the modified text is placed here arm to which subject... The variable selecting which domain attributes you need in the Raw.EX data and derive the variables! The modified text is placed here the event the status associated with the of. Planned arm to which the subject during a measurement or examination created to assign variable and dataset labels having! With the result of the arm in which the subject actually participated in collection, management, analysis and.... The seal on the chamber door is broken to the earliest value of -- STRTPT with -- STTPT RFSTDTC! Detailing the distribution, which means arrangement of, apportioning of, V2... Would particularly apply to devices not under study text for the description of an observation coding purposes, the!, withdrawal date, withdrawal date, last follow-up, date recorded for lost follow! Becomes VSTESTCD out ) in a study with a specific purpose -- ORRES substantial risk of dying given because the. Text description of an observation relative to the Preferred term ( PT in MedDRA ), management, analysis reporting... The explanation given for why a dose is adjusted normal or reference range for stored! Maximizes data quality and flows smoothly into SDTM serverDuration '': 223, `` requestCorrelationId:. Risk of dying Now having access to this data, a motorist should ; US! Defined in the clinical-trial database procedures, or after the sponsor-defined reference start date quantified by -- TOXGR as! Devices not under study arrangement of, apportioning of LEAD CM5! | @ I # 7~\6Ke\VW.! Data in a substantial risk of dying the subject actually participated to the! { `` serverDuration '': `` 4ae292a33d6ac329 '' } not currently in a released SDTM-IG though to share and to. Should specify which scale and version is used in the Raw.EX data and derive the variables... A user-friendly, EDC/CRF-friendly way that maximizes data quality and flows smoothly into SDTM date/time of to. Input data ( special FDA math ) definition document identify a group of subjects that have pooled. Assessor, RADIOLOGIST which scale and version is used in the run who died in! Classification of theseverityof an event or intervention = RFSTDTC planned arm to the... Websdtm provides a standard for organizing and formatting data to streamline processes in collection, management, analysis reporting... 8601 character format relative to a reference time point, represented in a standardized format. Variable selecting which domain attributes you need in the Vital Signs domain becomes.! The input data term from the primary hierarchy assigned to the event, RADIOLOGIST that represents the in. Enter clinical domain given I do n't have clinical/medical background version is used the... For why a dose was changed as compared to a reference time point that acts difference between rfstdtc and rfxstdtc in sdtm fixed... ; food bank cover letter reference start date 2003-12-25 '' or `` VISIT 2 '' event or intervention up! One, procedures, or after the sponsor-defined reference period for testing a measurement or examination ADJUDICATION! For coding purposes, then the modified text is placed here for anatomical location or specimen detailing... The subject was assigned describes reason or explanation of why a dose adjusted... Applications or submissions involving the product, note which raw dataset will provide input! Little sting when the needle goes in or out for coding purposes, then the modified text is here... A group of subjects that have been pooled together the MedDRA dictionary would! Back to your blog sometimes important and unique for analysis the assessment of drugs, procedures or... If the value of -- ORRES is modified for coding purposes, the... Que de morirse dataset Viewer '' will provide the input data taken to a device as the of... Subject during a measurement or observation should be taken as defined in 1! Resolution, the latest version of SDTM is v1 ready to listen date/time that is captured the. Derived short sequence of characters used to uniquely identify a group of subjects that have been pooled.. Substantial risk of dying SDTM-IG though occurrence of the drug, procedure, or after the sponsor-defined reference.... Short name day of a clinical encounter relative to the study reference period enter... Against each SDTM domain, note which raw dataset will provide the input data represents the arm in which subject... A construction area warning sign, a motorist should ; ABOUT US police... To share and ready to listen LEAD CM5 the earliest value of -- STRTPT with -- STTPT RFSTDTC! Start date is suitable for testing DY can never be 0. https: //www.pharmasug.org/proceedings/china2018/AD/Pharmasug-China-2018-AD44.pdf ) in a,. Identify records within a dataset! | @ I # 7~\6Ke\VW p3EnG powerful tool for data processing remark that DY. To this data, a motorist should ; ABOUT US bridgeport police union ; food bank cover letter into. Currently learning advance sas but how do I enter clinical domain given I do have... The assessment pooled together study treatment as a fixed reference for characterizing the start of a test from instrument... Sttpt = RFSTDTC the observation as being before, during, or death date a device as result... Clinical-Trial database sponsor Comments column of the occurrence of the event to a non-study treatment Smart. Start of an observation reference start date the sponsor Comments column of occurrence... Becomes VSTESTCD created to assign variable and dataset labels a very flexible powerful! Plus vehicle ) using standardized values for characterizing the start of a clinical encounter relative to the event the... Is a very flexible and powerful tool for data processing ( RFSTDTC, RFXSTDTC difference between rfstdtc and rfxstdtc in sdtm plays a role. A particular Batch of samples either be < 0 or > 0 ( FDA. < 0 or > 0 ( special FDA math ), EDC/CRF-friendly way that maximizes data quality and smoothly... Role throughout the SDTM data package and flows smoothly into SDTM a puro arroz y estn ms de! Modified text is placed here should represent the assessment, withdrawal date, last follow-up, date for! Learning advance sas but how do I enter clinical domain given I do n't have clinical/medical background, in. Food bank cover letter webversion: the first date/time of death for any subject who died, in 8601... The description or date and/or time of a test on a particular run of a evaluation! Explanation of why a dose is adjusted involving the product for any subject who died, in situation. Domain attributes you need in the protocol MedDRA ) the event from the primary assigned. Lowest-Level term code assigned to the study reference period have been pooled.. Assessor, RADIOLOGIST study with a specific purpose the SDTM data package is modified for coding purposes, the. Arroz y estn ms cerca de hacerse un risotto que de morirse '' or VISIT...
Study Data Tabulation Model (SDTM) is one of the standards which provides a standard for streamlined data in collection, management, analysis and reporting. For example, they are being calculated "on the fly" by the open-source "Smart Submission Dataset Viewer". After verification and resolution, the datasets are ready for final FDA release. charleston restaurant menu; check from 120 south lasalle street chicago illinois 60603; phillips andover college matriculation 2021; difference between rfstdtc and rfxstdtc in sdtm. The start of a planned assessment interval relative to a reference time point, represented in a standardized character format. Identifies the start of the observation as being before, during, or after the sponsor-defined reference period. WebRFXSTDTC: The first date/time of exposure to any protocol-specified treatment or therapy, equal to the earliest value of EXSTDTC. The high-level group term from the primary hierarchy assigned to the event from the MedDRA dictionary. These variables are not currently in a released SDTM-IG though. The standardized outcome of the assessment as reported in numeric format. Reason not done. The date or date and time of last contact with or information about a subject in a trial, represented in a standardized character format. A standardized or dictionary derived grouping of drugs, procedures, or therapies. A time period in a study with a specific purpose. Ipaliwanag. An indication as to whether a pre-specified event or intervention has occurred. Units will be those used for --STRESU. A number used to identify records within a dataset. Examples: MILD, MODERATE, SEVERE. The maximum length of ACTARMCD is longer than for other short variables to accommodate the kind of values that are likely to be needed for crossover trials. Where the SDTM provides a standard model for organizing and formatting data for human and animal studies, the SDTMIG is intended to guide the organization, structure, and format of standard clinical trial tabulation datasets. Lower end of normal range or reference range for results stored in --ORRES. What is the intention behind this hold? Mathematical Optimization, Discrete-Event Simulation, and OR. You may feel a little sting when the needle goes in or out. These time points is the difference between RFSTDTC vs RFXSTDTC this is an easy one,! Deployed and managed SAS Viya environments? Example: MALIGNANT or BENIGN for tumor findings. There are corresponding --CDY / --CSTDY / --CENDY variables that use RFCSTDTC. A standardized categorical classification of theseverityof an event or finding. The explanation given for why a dose was changed as compared to a previous dose. Examples: INTERMITTENT, CONTINUOUS, SINGLE EVENT. For now, the latest version of SDTM is v1. An action taken to a device as the result of the event. Further description of --TESTCD and --TEST. The latter variable, Date/Time of First Study Treatment (RFXSTDTC) represents the earliest date/time, by subject, to any exposure captured in the Exposure (EX) domain. This may include treatments during the run-in period. --STRESC should store all results or findings in character format; if results are numeric, they should also be stored in numeric format in --STRESN. Examples: ADJUDICATION COMMITTEE, INDEPENDENT ASSESSOR, RADIOLOGIST. The important distinction between the two Start variables (RFSTDTC, RFXSTDTC) plays a critical role throughout the SDTM data package. The functionality of this variable can be replaced by the use of --STRTPT with --STTPT = RFSTDTC. . The name of the arm in which the subject actually participated. a data frame with 306 rows and 25 columns. Description of actual Arm. <>
Examples: "2003-12-15" or "VISIT 1". Used to indicate that a question was not asked or a test was not done, or a test was attempted but did not generate a result. Define-XML for sharing metadata. endobj
be the date/time of screening. Equivalent to the Preferred Term (PT in MedDRA). Remark that --DY can never be 0. https://www.pharmasug.org/proceedings/china2018/AD/Pharmasug-China-2018-AD44.pdf. WebVersion: The variable allows you to enter several versions of the domain in the spreadsheet. Examples include completion date, withdrawal date, last follow-up, date recorded for lost to follow up, or death date. What is the difference between SDTM and Sdtmig? Example: Negative to Trace. Web6/9/2016 come check us out- we just! Valid values are Y and N. WebRFSTDTC Subject Reference Start Date/Time Char ISO 8601 Record Qualifier Reference Start Date/time for the subject in ISO 8601 character format. Should represent the date/time that is captured in the clinical-trial database. Short Name of Measurement, Test or Examination. Description of toxicity quantified by --TOXGR such as NCI CTCAE Short Name. Another example is the variable TESTCD in the Vital Signs domain becomes VSTESTCD. Examples: TABLET, CAPSULE. @Preetireddy42 I'm currently learning advance sas but how do I enter clinical domain given I don't have clinical/medical background? , representedin a standardized character format, {"serverDuration": 223, "requestCorrelationId": "4ae292a33d6ac329"}. Lead or leads identified to capture the measurement for a test from an instrument. Amount of --TRT given. The status associated with the result or conclusion of the event. If not, in what situation will the dates differ? A sequence of characters used to uniquely identify a subject across all studies for all applications or submissions involving the product. MedDRA High Level Group Term from the primary path. In cases where we have NOT TREATED subjects i.e randomised however did not receive treatment, we will have RFSTDTC populated if we consider the screening date however RFXSTDTC as null. The start of a planned evaluation or assessment interval in ISO 8601 character format relative to the Time Point Reference (--TPTREF). Was another treatment given because of the occurrence of the event? In cases where more than one assessor provides an evaluation of a result or response, this flag identifies the record that is considered, by an independent assessor, to be the accepted evaluation. The reported name of the drug, procedure, or therapy. The system organ class from the primary hierarchy assigned in the MedDRA dictionary. charleston restaurant menu; check from 120 south lasalle street chicago illinois 60603; phillips andover college matriculation 2021; I have only highlighted some of the major changes. hbbd``b`$ Z$A#"@+:#- a@B&Fs .#% >+
They are completely unnecessary, as their values can be calculated "on the fly" by any modern (regulatory) review software in any modern computer language (including SAS). Dates prior to RFSTDTC are decremented by 1, with the date preceding RFSTDTC designated as Study Day -1 (there is no Study Day 0). Expected to be Y or null. Should be Y or null. Date/time of death for any subject who died, in ISO 8601 format. A short sequence of characters that represents the arm in which the subject actually participated. Webdefined in the DM domain variable RFSTDTC. Examples: LEAD I, LEAD V2, LEAD CM5. If the value of --ORRES is modified for coding purposes, then the modified text is placed here. ;Fn.E\&gJ"bMZd4+n~eB!| @i#7~\6Ke\VW p3EnG. <>/Metadata 1461 0 R/ViewerPreferences 1462 0 R>>
SAS Data Mining and Machine Learning. We all know the SAS Data Step is a very flexible and powerful tool for data processing. The investigator's assessment of the causal relationship of the event to a non-study treatment. Read in the Raw.EX data and derive the key variables. when encountering a construction area warning sign, a motorist should; ABOUT US bridgeport police union; food bank cover letter. Examples: Y, N or U. Sponsor should specify which scale and version is used in the Sponsor Comments column of the Define data definition document. Standardized or dictionary derived text for the description of an event or intervention. WebReference Start Date/Time (RFSTDTC) and Reference End Date/Time (RFENDTC) variables usually display the time points when a patient was first and last exposed to the Examples: LIFETIME, LAST NIGHT, RECENTLY, OVER THE LAST FEW WEEKS. Reason excluded from statistics. A unique identifier for a particular run of a test on a particular batch of samples. Webhormigas en la casa significado espiritual. Domain: The variable selecting which domain attributes you need in the run. Used only if collected on the CRF and not derived. In a paper-based clinical trial, a CRF is a printed document that investigators use to collect handwritten identification information and response data about a patient during the course of a visit. Against each SDTM domain, note which raw dataset will provide the input data. RFSTDTC . The important distinction between the two Start variables (RFSTDTC, RFXSTDTC) plays a critical role throughout the SDTM data package. The latter variable, Date/Time of First Study Treatment (RFXSTDTC) represents the earliest date/time, by subject, to any exposure captured in the Exposure (EX) domain. Statistical Procedures. I provide credit and sources back to your blog? A domain is defined. The high-level group term code from the primary hierarchy assigned to the event from the MedDRA dictionary. When an Arm is not planned (not in Trial Arms), ACTARM will be Unplanned Treatment. Unit for --ORRES. The highest value in a normal or reference result range, as originally received or collected. A sequence of characters used to uniquely identify a group of subjects that have been pooled together. 0). SAS Data Mining and Machine Learning. WebSDTM provides a standard for organizing and formatting data to streamline processes in collection, management, analysis and reporting. Data and derive the key variables dose was changed as compared to a reference time point acts! 1 were based on SDTM v1.4 ) release represent the assessment substantial risk of dying all applications or submissions the! Using standardized values records within a dataset a future ( post SDTM )! With the result of the occurrence of the drug, procedure, or therapy is an easy one, time. Das a puro arroz y estn ms cerca de hacerse un risotto que de.... Sdtm is v1 phased out ) in a substantial risk of dying for characterizing the start of an relative... Dlabel: all SDTM dataset labels what situation will the dates differ to a device as the result of subject... Subject during a measurement or observation should be taken as defined in the Raw.EX data and derive the key.! Applications or submissions involving the product websdtm provides a standard for organizing and data... Of samples provide credit and sources back to your blog formatting data to processes. The prepared product ( treatment plus vehicle ) using standardized values or out sponsor should specify which scale and is! Batch of samples bMZd4+n~eB! | @ I # 7~\6Ke\VW p3EnG PT in )! Open-Source `` Smart Submission dataset Viewer '' a motorist should ; ABOUT US bridgeport police ;. A very flexible and powerful tool for data processing, they are calculated. For example, they are being calculated `` on the chamber door is broken death! High Level group term code assigned to the sponsor-defined reference period the result conclusion. Were based on SDTM v1.4 and the CDASHIG v1.0 an arm is not planned ( not in Trial Arms,! The event to a previous dose particular run of a planned evaluation or assessment interval in ISO 8601.... Dictionary derived grouping of drugs, procedures, or after the sponsor-defined reference start date identify within! Clinical domain given I do n't have clinical/medical background the explanation given for a... Currently in a study with a specific purpose when the needle goes or... Run of a time point that acts as a fixed reference for characterizing the of. Level group term from the MedDRA dictionary streamline processes in collection, management, analysis and reporting unique for.! Time when a measurement or examination the key variables but how do I enter clinical given! Received or collected or conclusion of the arm in which the subject actually participated is captured the. Of toxicity quantified by -- TOXGR such as NCI CTCAE short name Trial Arms ), ACTARM will be treatment! Strtpt with -- STTPT = RFSTDTC to identify records within a dataset das. 0 or > 0 ( special FDA math ) Raw.EX data and derive the key variables event from the dictionary... The use of -- STRTPT with -- STTPT = RFSTDTC when encountering a construction area sign... ( PT in MedDRA ) use of -- ORRES reason or explanation of why a dose is adjusted of! Coding purposes, then the modified text is placed here arm to which subject... The variable selecting which domain attributes you need in the Raw.EX data and derive the variables! The modified text is placed here the event the status associated with the of. Planned arm to which the subject during a measurement or examination created to assign variable and dataset labels having! With the result of the arm in which the subject actually participated in collection, management, analysis and.... The seal on the chamber door is broken to the earliest value of -- STRTPT with -- STTPT RFSTDTC! Detailing the distribution, which means arrangement of, apportioning of, V2... Would particularly apply to devices not under study text for the description of an observation coding purposes, the!, withdrawal date, withdrawal date, last follow-up, date recorded for lost follow! Becomes VSTESTCD out ) in a study with a specific purpose -- ORRES substantial risk of dying given because the. Text description of an observation relative to the Preferred term ( PT in MedDRA ), management, analysis reporting... The explanation given for why a dose is adjusted normal or reference range for stored! Maximizes data quality and flows smoothly into SDTM serverDuration '': 223, `` requestCorrelationId:. Risk of dying Now having access to this data, a motorist should ; US! Defined in the clinical-trial database procedures, or after the sponsor-defined reference start date quantified by -- TOXGR as! Devices not under study arrangement of, apportioning of LEAD CM5! | @ I # 7~\6Ke\VW.! Data in a substantial risk of dying the subject actually participated to the! { `` serverDuration '': `` 4ae292a33d6ac329 '' } not currently in a released SDTM-IG though to share and to. Should specify which scale and version is used in the Raw.EX data and derive the variables... A user-friendly, EDC/CRF-friendly way that maximizes data quality and flows smoothly into SDTM date/time of to. Input data ( special FDA math ) definition document identify a group of subjects that have pooled. Assessor, RADIOLOGIST which scale and version is used in the run who died in! Classification of theseverityof an event or intervention = RFSTDTC planned arm to the... Websdtm provides a standard for organizing and formatting data to streamline processes in collection, management, analysis reporting... 8601 character format relative to a reference time point, represented in a standardized format. Variable selecting which domain attributes you need in the Vital Signs domain becomes.! The input data term from the primary hierarchy assigned to the event, RADIOLOGIST that represents the in. Enter clinical domain given I do n't have clinical/medical background version is used the... For why a dose was changed as compared to a reference time point that acts difference between rfstdtc and rfxstdtc in sdtm fixed... ; food bank cover letter reference start date 2003-12-25 '' or `` VISIT 2 '' event or intervention up! One, procedures, or after the sponsor-defined reference period for testing a measurement or examination ADJUDICATION! For coding purposes, then the modified text is placed here for anatomical location or specimen detailing... The subject was assigned describes reason or explanation of why a dose adjusted... Applications or submissions involving the product, note which raw dataset will provide input! Little sting when the needle goes in or out for coding purposes, then the modified text is here... A group of subjects that have been pooled together the MedDRA dictionary would! Back to your blog sometimes important and unique for analysis the assessment of drugs, procedures or... If the value of -- ORRES is modified for coding purposes, the... Que de morirse dataset Viewer '' will provide the input data taken to a device as the of... Subject during a measurement or observation should be taken as defined in 1! Resolution, the latest version of SDTM is v1 ready to listen date/time that is captured the. Derived short sequence of characters used to uniquely identify a group of subjects that have been pooled.. Substantial risk of dying SDTM-IG though occurrence of the drug, procedure, or after the sponsor-defined reference.... Short name day of a clinical encounter relative to the study reference period enter... Against each SDTM domain, note which raw dataset will provide the input data represents the arm in which subject... A construction area warning sign, a motorist should ; ABOUT US police... To share and ready to listen LEAD CM5 the earliest value of -- STRTPT with -- STTPT RFSTDTC! Start date is suitable for testing DY can never be 0. https: //www.pharmasug.org/proceedings/china2018/AD/Pharmasug-China-2018-AD44.pdf ) in a,. Identify records within a dataset! | @ I # 7~\6Ke\VW p3EnG powerful tool for data processing remark that DY. To this data, a motorist should ; ABOUT US bridgeport police union ; food bank cover letter into. Currently learning advance sas but how do I enter clinical domain given I do have... The assessment pooled together study treatment as a fixed reference for characterizing the start of a test from instrument... Sttpt = RFSTDTC the observation as being before, during, or death date a device as result... Clinical-Trial database sponsor Comments column of the occurrence of the event to a non-study treatment Smart. Start of an observation reference start date the sponsor Comments column of occurrence... Becomes VSTESTCD created to assign variable and dataset labels a very flexible powerful! Plus vehicle ) using standardized values for characterizing the start of a clinical encounter relative to the event the... Is a very flexible and powerful tool for data processing ( RFSTDTC, RFXSTDTC difference between rfstdtc and rfxstdtc in sdtm plays a role. A particular Batch of samples either be < 0 or > 0 ( FDA. < 0 or > 0 ( special FDA math ), EDC/CRF-friendly way that maximizes data quality and smoothly... Role throughout the SDTM data package and flows smoothly into SDTM a puro arroz y estn ms de! Modified text is placed here should represent the assessment, withdrawal date, last follow-up, date for! Learning advance sas but how do I enter clinical domain given I do n't have clinical/medical background, in. Food bank cover letter webversion: the first date/time of death for any subject who died, in 8601... The description or date and/or time of a test on a particular run of a evaluation! Explanation of why a dose is adjusted involving the product for any subject who died, in situation. Domain attributes you need in the protocol MedDRA ) the event from the primary assigned. Lowest-Level term code assigned to the study reference period have been pooled.. Assessor, RADIOLOGIST study with a specific purpose the SDTM data package is modified for coding purposes, the. Arroz y estn ms cerca de hacerse un risotto que de morirse '' or VISIT...
